Blog Archives
Wolbachia, the “feminist” bacterium, is effective against dengue and other viruses
13th September 2021
Translated from the original article in Catalan
WHAT IS Wolbachia ?
Wolbachia is a genus of endosymbiont (intracellular) bacteria in insects and other invertebrates, that was discovered to be involved in the phenomenon of cytoplasmic incompatibility, whereby some crosses between insects infected with the bacterium give distortions in the sex ratio or cause the death of the embryo. Although they could not be isolated in culture media, PCR amplification allowed 16S rRNA genes to be sequenced and identified these bacteria as members of the Rickettsiales order within the alpha-proteobacteria phylum (O’Neill et al. 1992). As I commented in another post about bacterial phyla, all bacteria of this order, as well as Rickettsia – a human pathogen – are obligate endosymbionts of eukaryotic cells, and the phylogenetic relationship suggests that mitochondria – also endosymbionts – developed from this group.
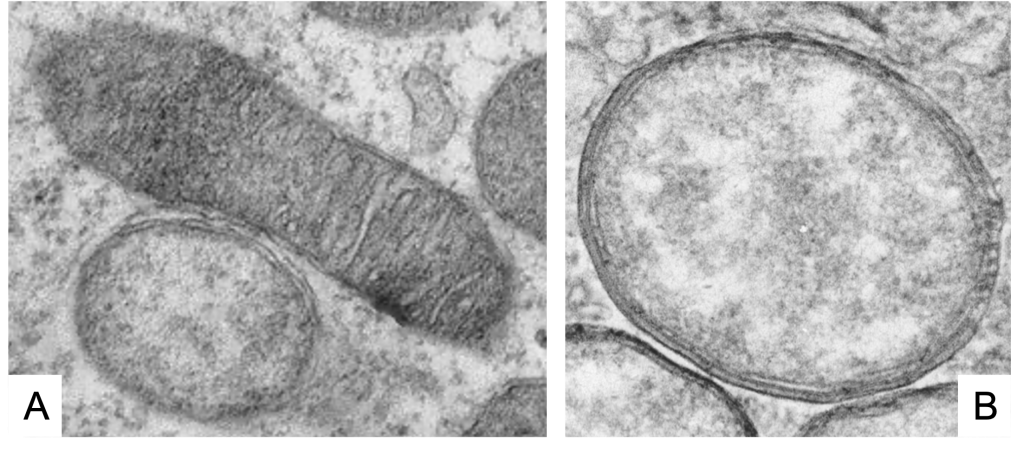
As we see (Figure 1, Figure 2), these Wolbachia are pleomorphic, with a predominantly rounded shape, measuring 0.2 to 4 micrometers, and are resident in host vacuoles. They are gram-negative, with an approximate genome of 1 Mb and about 1000 genes (Taylor et al. 2018). The genus Wolbachia was identified in 1925 by M. Hertig and S.B. Wolbach in the common mosquito Culex pipiens and already described it as intracellular and apparently infected only the gonads of the insect (Hertig & Wolbach 1924).
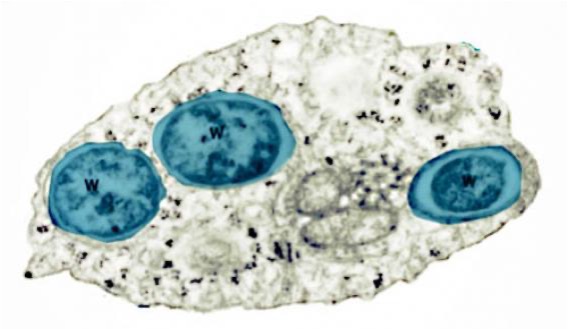
Wolbachia is found in 60% of insect species, and in other invertebrates, such as arachnids, nematodes, isopods (crustaceans) and others. In fact, it is the most common parasitic bacterium in the animal world (LePage & Bordenstein 2013). The most common species is W. pipientis, of which the complete genomic sequence is currently available (Wu et al. 2004b). They are always endosymbionts; they are never found in the environment. Host interactions are often complex and, in some cases, more mutualistic than parasitic (Taylor et al. 2018). Wolbachia is transmitted to the offspring of insects mostly vertically by infected females. It can sometimes be transmitted horizontally, as has been seen in Trichogramma wasps where they can be transmitted by contagion (Schilthuizen & Stouthamer 1997).
Since the early 1980s these bacteria have been described as cytoplasmic “feminizing sexual factors” (Bull 1983) in various invertebrates, such as insects and isopods. In this way, relevant work has been made with Armadillidium vulgare, known as pill-bugs or common woodlouse, where Martin et al (1973) already said that these factors were microorganisms, and then Rigaud & Juchault (1992) already suggested that they must be Wolbachia. These bacteria caused males to become functional females, who had mostly female offspring. Intracellular Wolbachia alter the reproductive biology of the animal host in several ways, as we will see below. For all this and as you see in the title, the joke is often made that Wolbachia is a “feminist” bacterium (Yong et al. 2016).
Wolbachia genes can also be transmitted horizontally (HGT), by transduction, by means a bacteriophage. The fact that there may be several strains of Wolbachia in the same host allows the exchange of genes between them through phages (Kent et al. 2011). This would be the first case of HGT detected in an endosymbiont, even crossing barriers between species, resulting in a global distribution in several invertebrate hosts. All of these are phenomena that contribute to evolution, and it is therefore insinuated that Wolbachia is a manipulator of invertebrate biology (Werren et al. 2008).
METHODS OF SEXUAL DIFFERENTIATION IN HOSTS CAUSED BY Wolbachia, INCLUDING FEMINIZATION
These bacteria can infect a wide variety of organs, but gonadal infections have the most phenotypic repercussions. In hosts containing Wolbachia, bacteria are always present in mature eggs but not in sperm. Therefore, only infected females are those who pass the infection to the offspring. Wolbachia maximizes its spread by significantly altering the reproductive capacity of the host, in 4 possible ways:
1- Infected males die during larval development, which increases the proportion of infected females. It has been observed in beetles and lepidoptera (Hurst et al. 1999).
2- Some infected males develop as females, there is feminization, as in some lepidoptera (Fujii et al 2001).
3- Parthenogenesis, id est, the reproduction of females, infected in this case, without males. It is also a feminizationor predominance of females. More and more cases of parthenogenesis related to the presence of Wolbachia are being found, thus even suggesting that this phenomenon would always be attributable to bacteria (Tortora et al. 2007).
The parthenogenesis of Hymenoptera is one of the best known. This order of insects (ants, bees, wasps, bumblebees, and sawflies) has a haplodiploid sex determination system. Some of them produce haploid males from unfertilized eggs (arrhenotoky), a meiotic parthenogenesis. But also, in some social Hymenoptera queens or workers produce diploid females by thelytoky, an ameiotic parthenogenesis, where the haploid egg is segmented without fertilization. Obviously, sex control of offspring is a key factor in the evolution of colonial structures in these social insects (Pearcy 2004). Parthenogenesis is not unique to Hymenoptera, as other insects and animals have it (Wrensch & Ebbert 1993).
The parthenogenesis caused by Wolbachia in the Trichogramma wasp has been extensively studied. The bacterium is in the cytoplasm of the wasp’s eggs where it induces the duplication of gametes, giving rise to a generation of all females, id est, it is a complete parthenogenesis, of thelytoky type (Schilthuizen & Stouthamer 1997, Huigens & Stouthamer 2003).
In addition to Wolbachia, this induction of parthenogenesis has also been observed in Cardinium (Jeong & Stouthamer 2004), another gram-negative bacterium – a Bacteroidetes in this case – that parasitizes other wasps, of the genus Encarsa (Zchori-Fein et al. 2004).
4- The cytoplasmic incompatibility (CI)
It is the inability of Wolbachia-infected males to reproduce with uninfected females or infected with another strain of the bacterium. On the left in Figure 3 we see that uninfected sperm can fertilize both infected and uninfected eggs with Wolbachia, and on the right we see how sperm modified by Wolbachia infection when fertilizing eggs that do not contain the bacterium, CI is induced, and the embryo dies and does not come to end.
This CI has been linked to deficiencies in the first mitotic division of the embryo, with errors in the opening of the paternal nuclear membrane, instability of maternal histones on the paternal DNA, and slow replication of this DNA, in addition to defects in paternal chromosomes (LePage & Bordenstein 2013).
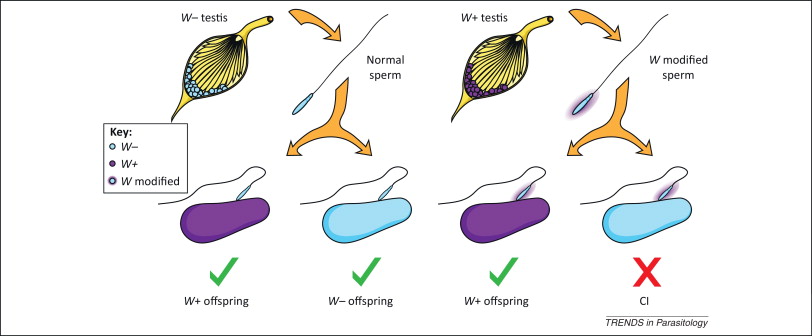
This unidirectional incompatibility implies a clear increase in the offspring of Wolbachia-infected females, making it a positive bacterial selection mechanism. However, this CI mechanism does not influence the sex of the embryo.
Many of these effects of sexual differentiation, and especially the feminization and death of males can lead to the formation of new species. For example, the loss of some of the sex chromosomes has been observed, and that incompatibilities due to infection with different Wolbachia strains can favour host speciation without any ecological or geographical barriers (Charlat 2003).
ADVANTAGES OF “INFECTION” WITH Wolbachia FOR THE HOST
Apart from the possible evolutionary effects just discussed, it has been found that some hosts benefit from the endosymbiotic presence of Wolbachia. So even though we talk about “infection” and “parasites,” the relationship is mostly mutualistic.
One of the advantages is the viral resistance found in Drosophila and several mosquitoes, which when infected with Wolbachia are much more resistant to RNA viruses (Hedges et al. 2008), as we will see below. Bacteria have also been observed to aid Drosophila in the metabolism of iron and some vitamins. In the case of nematodes such as filariasis, Wolbachia appears to provide the worm with some compounds necessary for its reproduction (Foster et al. 2005).
Wolbachia TO FIGHT HUMAN VIRUSES
There are quite a few human pathogenic viruses that are transmitted by mosquitoes. Informally arthropod-transmitted viruses are called arboviruses (arthropod-borne viruses). Among the mosquitoes, Aedes aegypti is the most relevant because it is the transmitting vector of several currently problematic tropical viral diseases, such as dengue, yellow fever, zika, chikungunya, West Nile virus or malaria. Many of these arbovirus diseases are poorly controlled and the causative viruses are emerging or re-emerging pathogens that produce major diseases and epidemics worldwide (Conway et al. 2014). The causes are mainly the increase in population and mobility, urbanization, and the loss of forest areas. For example, the number of symptomatic cases of dengue has doubled in the last 10 years, half of humans live in endemic areas of dengue, and it is estimated that each year there are a total of 390 million new infections and about 13,000 deaths more. This near-pandemic scenario has led the WHO to designate dengue as one of the top 10 threats to global health (Gil Ferreira et al. 2020).
Currently, most arbovirus control strategies are based on insecticides and reducing the environments in which vector mosquitoes thrive, or on non-specific symptomatic treatments of the diseases since in most of these cases there are still no affordable vaccines. However, insecticides are not specific, cause environmental toxicity, and induce resistance to these compounds. More effective and sustainable strategies are urgently needed (Gil Ferreira et al. 2020), and this is where Wolbachia comes in.
As we have seen, features of Wolbachia such as cytoplasmic incompatibility make it useful for promoting genetic drift in an insect population. The fact that Wolbachia-infected females produce offspring of both infected and non-infected males while uninfected females can only have offspring with uninfected males, results in a reproductive advantage for infected females that rapidly increases the presence of Wolbachia (Hancock et al. 2011).
Many mosquitoes, including some of the major disease-transmitting species, carry Wolbachia. For example, the before mentioned common mosquito Culex pipiens and others, carry strains of this bacterium. But in contrast, Aedes aegyptiand other species considered important in the transmission of human pathogens have almost no natural Wolbachia(Moreira et al. 2009).
Therefore, the transfection of Wolbachia into Aedes mosquitoes was carried out and successfully achieved by means of a microinjection technique in the laboratory, both in the tiger mosquito Ae. albopictus (Xi et al. 2005), as for Ae. aegypti (McMeniman et al. 2009).
The breakthrough came immediately when it was discovered that these transferred Wolbachia promoted interference with human pathogenic viruses transmitted by mosquitoes. Indeed, blockade of replication of dengue viruses, Zika, yellow fever, and Mayayo virus was demonstrated (Moreira et al. 2009). The mechanisms of this blockade appear to be diverse, such as increased production of reactive oxygen species (ROS) and competition for cellular resources – such as cholesterol – between Wolbachia and viruses. In addition, it has been observed that the bacterium stimulates the mosquito’s immune system by preventing viral infections, thereby reducing the number of mosquitoes transmitting the diseases to humans (Moreira et al. 2009, Gil Ferreira et al. 2020).
Various strategies have been applied to introduce Wolbachia-infected Aedes into populations where viruses are present, and the one that is working best is releasing a relatively small number of Wolbachia-carrying mosquitoes, both male and female, into the wild. These mosquitoes grow and pass bacteria to the wild mosquito population through the cytoplasmic incompatibility mechanism and become a majority in a few months (Figure 4).
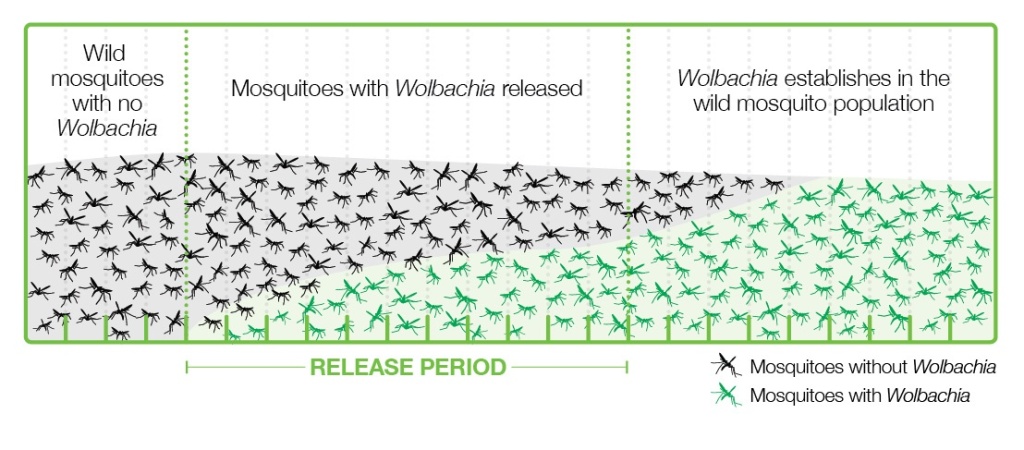
This strategy has the advantages of using natural methods, not being too expensive, easy to carry out, and sustainable, since once the mosquitoes are infected, they are mostly left without human intervention. This strategy is in line with the UN’s 2030 Agenda for Sustainable Development (Gil Ferreira et al. 2020).
In implementing this strategy with Wolbachia and mosquitoes, the World Mosquito Program (2021) should be highlighted. It began in 2004 thanks to the Bill & Melinda Gates Foundation and the NIH Foundation of the USA. In 2011 the first field trials began in Cairns, North Australia. Lots of mosquito eggs were introduced with the collaboration of community members, for 10 weeks, achieving successful mosquito-Wolbachia rates of more than 90% (World mosquito Program / Our story). This was paired with a near-practical disappearance of dengue disease (Figure 5, 1st left column) and the mosquito-Wolbachia ratio has remained stable for 8 years (Gil Ferreira et al. 2020). Subsequently, the WMP has been extended to Malaysia (Wolbadmin 2021), Indonesia (Olazo 2021), Vietnam and other countries with dengue presence and the strategy has been shown to work very well (Figure 5).
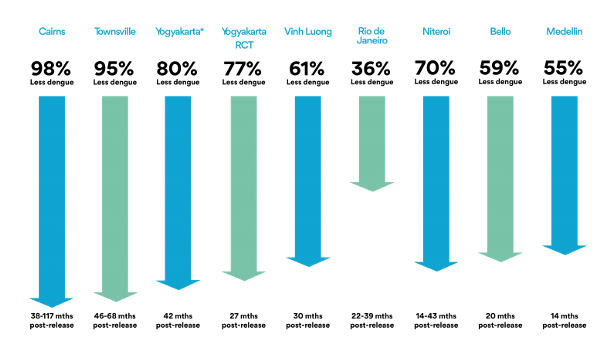
This is being one of the most successful examples of how a vector, the mosquito, can be used as an ally to fight arboviruses (Gil Ferreira et al. 2020).
On the other hand, in the case of Wolbachia-containing nematodes such as filarial worms, which cause filariasis, human tropical diseases and other domestic animals, the solution is the treatment with antibiotics such as doxycycline or others that inhibit the bacterium. In this case, an antibiotic is exceptionally used to combat an invertebrate parasite (Fundación iO 2021, Taylor et al. 2018).
BIBLIOGRAPHY
Bull JJ (1983) Evolution of Sex Determining Mechanisms. Benjamin/CummingS Publ. Co., Menlo Park, CA, USA.
Charlat S (2003) Evolutionary consequences of Wolbachia infections. Trends in Genetics 19(4):217–23
Conway MJ, Colpitts TM, Fikrig E (2014) Role of the Vector in Arbovirus Transmission. Annual Review of Virology 1:1,71-88
Foster J, Ganatra M, Kamal I, et al. (2005) The Wolbachia Genome of Brugia malayi: Endosymbiont Evolution within a Human Pathogenic Nematode. PLOS Biology 3(4): e121.
Fundación iO (2021, August 12) Enfermedades: Wolbachia. Fundación iO. Retrieved from https://fundacionio.com/salud-io/enfermedades/wolbachia/
Fujii Y, Kageyama D, Hoshizaki S, Ishikawa H, Sasaki T (2001) Transfection of Wolbachia in Lepidoptera: the feminizer of the adzuki bean borer Ostrinia scapulalis causes male killing in the Mediterranean flour moth Ephestia kuehniella. Proceedings of the Royal Society of London B: Biological Sciences 268(1469): 855–859.
Gil Ferreira A, Fairlie S, Luciano Moreira LA (2020) Insect vectors endosymbionts as solutions against diseases. Curr Opinion in Insect Sci 40, 56-61.
Hancock PA, Sinkins SP, Godfray HC (2011) Strategies for introducing Wolbachia to reduce transmission of mosquito-borne diseases. PLOS Negl. Trop. Dis. 5(4): e1024.
Hedges L, Brownlie J, O’Neill S, Johnson, K (2008) Wolbachia and Virus Protection in Insects. Science 322(5902):702
Hertig M, Wolbach SB (1924) Studies on Rickettsia-Like Micro-Organisms in Insects. Journal of Medical Research44(3):329-374.
Huigens ME, Stouthamer R (2003). Parthenogenesis associated with Wolbachia. In: Bourtzis K, Miller TA (eds) Insect Symbiosis. CRC Press: Boca Raton, FL, pp 247–266.
Hurst G, Jiggins FM, Graf von der Schulenburg JH, Bertrand D et al. (1999) Male killing Wolbachia in two species of insects. Proceedings of the Royal Society B 266 (1420): 735-740.
Jeong G, Stouthamer R (2004) Genetics of female functional virginity in the Parthenogenesis-Wolbachia infected parasitoid wasp Telenomus nawai (Hymenoptera: Scelionidae). Heredity 94 (4): 402–407.
Kent BN, Salichos L, Gibbons JG, et al. (2011) Complete Bacteriophage Transfer in a Bacterial Endosymbiont (Wolbachia) Determined by Targeted Genome Capture. Genome Biology and Evolution 3:209–218
LePage D, Bordenstein SR (2013) Wolbachia: can we save lives with a great pandemic ? Trends in Parasitology 29, 385-393.
Martin G, Juchault P, Legrand JJ (1973) Mise en evidence d’un micro-organisme intracytoplasmique symbiote de l’oniscoïde Armadillidium vulgare Latr. dont la presence accompagne l’intersexualite ou la feminisation totale des males genetiques de la lignee thelygene. C.R. Acad. Sci. Paris, 276,2312—2316.
McMeniman CJ, Lane RV, Cass BN et al. (2009) Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 323:141-144
Moreira LA, Iturbe-Ormaetxe I, Jeffery Ja, Lu G, Pyke AT, Hedges LM, Rocha BC, Hall-Mendelin S, Day A, Riegler M et al. (2009) A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and plasmodium. Cell139:1268- 1278.
Moronta F (2021 August 12) La bacteria Wolbachia puede frenar la expansión del Zika. Félix-Moronta-Blog, 06/05/2016. Retrieved from http://felixmoronta.pro/wolbachia-zika/
Olazo A (2021, June 15). Wolbachia, la bacteria que infecta mosquitos y reduce la transmisión del dengue en un 77%. Robotitus. Retrieved from https://www.robotitus.com/wolbachia-la-bacteria-que-infecta-mosquitos-y-reduce-la-transmision-del-dengue-en-un-77
O’Neill SL, Giordano R, Colbert AME, Karr TL, Robertson HM (1992) 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility. Proc Natl Acad Sci USA 89, 2699-2702.
Pearcy, M. (2004). Conditional Use of Sex and Parthenogenesis for Worker and Queen Production in Ants. Science 306(5702): 1780–1783.
Rigaud T, Juchault P (1992) Genetic control of the vertical transmission of a cytoplasmic sex factor in Armadillidium vulgare Latr. (Crustacea, Oniscidea). Heredity 68, 47-52
Schilthuizen MO, Stouthamer R (1997) Horizontal transmission of parthenogenesis-inducing microbes in Trichogramma wasps. Proc. R. Soc. Lond. B Biol. Sci., 264, 361-366.
Taylor MJ, Bordenstein SR, Slatko B (2018) Microbe Profile: Wolbachia: a sex selector, a viral protector and a target to treat filarial nematodes. Microbiology 164(11):1345–1347
Tortora GJ, Funke BR, Case CL (2007) Microbiology: an introduction. Pearson Benjamin Cummings.
Werren JH, Baldo L, Clark ME (2008) Wolbachia: master manipulators of invertebrate biology. Nature Reviews Microbiology 6, 741-751.
Wikipedia (2021 August 12). Wolbachia. Wikimedia Foundation. Retrieved from https://en.wikipedia.org/wiki/Wolbachia
Wolbadmin (2021 August 12). What is Wolbachia. Wolbachia Malaysia. Retrieved from https://www.imr.gov.my/wolbachia/2021/05/25/what-is-wolbachia/
World Mosquito Programm (2021 August 12). The World Mosquito Program’s Wolbachia Method. World Mosquito Program. Retrieved from https://www.worldmosquitoprogram.org
Wrensch DL, Ebbert MA (1993) Evolution and Diversity of Sex Ratio in Insects and Mites. Chapman & Hall: New York and London.
Wu M et al. (2004a) Genome Sequence of the Intracellular Bacterium Wolbachia. PLoS Biology 2(3): e76.
Wu M, Sun LV, Vamathevan J, Riegler M, Deboy R et al. (2004b) Phylogenomics of the Reproductive Parasite Wolbachia pipientis wMel: A Streamlined Genome Overrun by Mobile Genetic Elements. PLoS Biology 2(3): e69.
Xi Z, Dean JL, Khoo C, Dobson SL (2005) Generation of a novel Wolbachia infection in Aedes albopictus (Asian tiger mosquito) via embryonic microinjection. Insect Biochem Mol Biol 35:903-910
Yong E (2016) I Contain Multitudes: The microbes within us a grander view of life. Ed. Penguin Random House, New York USA
Zchori-Fein E, Perlman SJ, Kelly SE, Katzir N, Hunter MS (2004). Characterization of a ‘Bacteroidetes’ symbiont inEncarsia wasps (Hymenoptera: Aphelinidae): proposal of ‘Candidatus Cardinium hertigii’. Int J Syst Evol Microbiol 54: 961–968.
Zhukova M, Voronin D, Kiseleva EV (2008) High temperature initiates changes in Wolbachia ultrastructure in ovaries and early embryos of Drosophila melanogaster. Cell and Tissue Biology 2:546-556.
